A cluster of presumed, noninfectious endophthalmitis after intravitreal injection of bevacizumab: long-term follow-up
Main Article Content
Abstract
Purpose
To report the outcome of 5 consecutive cases of presumed, noninfectious endopththalmitis following intravitreal injection of bevacizumab (IVB).
Methods
Ten pre-loaded syringes of bevacizumab (1.25 mg/50 µL) furnished by a compounding pharmacy were injected intravitreally. Treatments were performed in the operating room by the same surgeon on 2 consecutive days.
Results
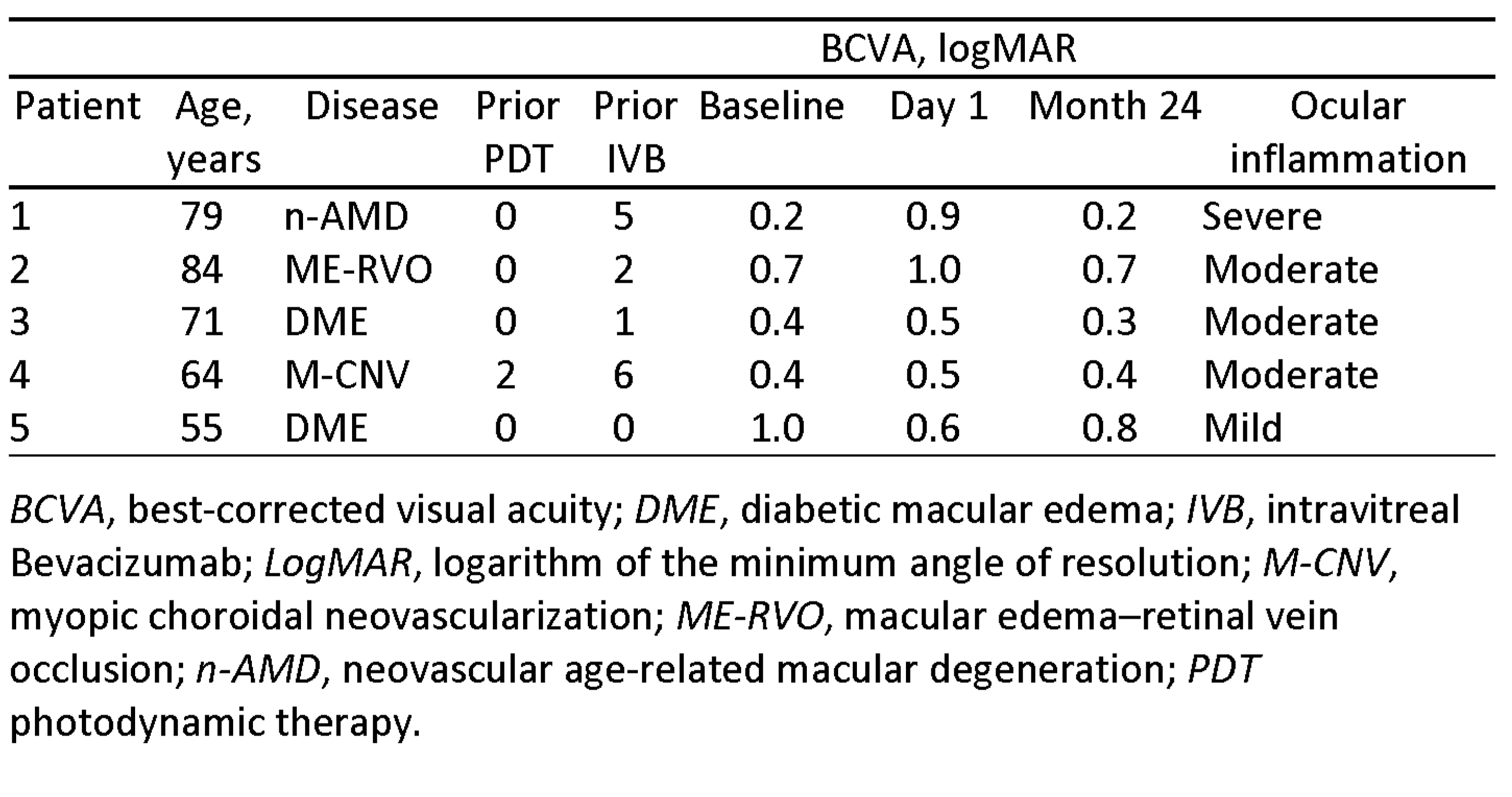
Of 10 eyes, 5 showed moderate to severe ocular inflammation within a few days of injection. All patients were treated in the same surgical session. Vitreous tap performed in the patient presenting with the most severe grade of inflammation was negative for bacteria and fungi. At the time of the vitreous biopsy, this patient was injected with vancomycin 1 mg/100 µL in the vitreous cavity. Other eyes with moderate inflammation received topical and systemic antibiotics and topical steroid treatment. Visual acuity returned to pre-endophthalmitis or better levels in all eyes within 1 month. The other 5 patients treated with IVB from the same batch in the other surgical session did not develop inflammation.
Conclusions
IVB can induce noninfectious endophthalmitis. The use of compounded syringes can explain clustering of the inflammation. We were unable to identify the reasons for the variable grade of inflammation we observed in our patients.
Downloads
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.