Outcomes of mitomycin C intravascular chemoembolization (MICE) in refractory corneal neovascularization after failed keratoplasty
Main Article Content
Abstract
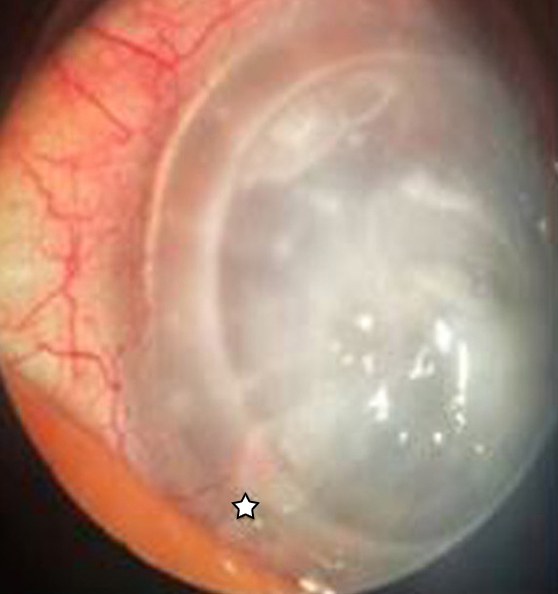
Corneal neovascularization is a determinant of corneal graft survival and preservation of immune privilege after keratoplasty. We report the outcomes in 2 patients with failed corneal grafts who underwent mitomycin C (MMC) intravascular chemoembolization (MICE) in the affected eye. A 30-year-old woman with failed penetrating keratoplasty (PK) in the right eye was started on prednisolone acetate eyedrops. Graft sutures were removed, and bevacizumab was injected subconjunctivally. The eye remained intermittently painful, and MICE was performed on the main feeding vessel, with regression of the vessels apparent within the first day following the procedure. The second case was a 40-year-old man who had a history of repaired penetrating injury in the left eye followed by failed PK. Prednisolone acetate eyedrops were initiated, and corneal sutures were removed. The patient failed to improve with three subconjunctival injections of bevacizumab. MICE was performed, but in this case neovascularization did not regress until 20 weeks post-procedure. MMC is thought to inhibit vascular endothelial cell proliferation, but its use in corneal injection is debated. In these cases, MICE was not associated with any concerning adverse events.
Downloads
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
Williams KA, Roder D, Esterman A, Muehlberg SM, Coster DJ. Factors predictive of corneal graft survival: report from the Australian Corneal Graft Registry. Ophthalmology 1992;99:403-14. DOI: https://doi.org/10.1016/S0161-6420(92)31960-8
Larkin D. Corneal transplantation for herpes simplex keratitis. Br J of Ophthalmol 1998;82:107-8. DOI: https://doi.org/10.1136/bjo.82.2.107
Dana MR, Qian Y, Hamrah P. Twenty-five–year panorama of corneal immunology: emerging concepts in the immunopathogenesis of microbial keratitis, peripheral ulcerative keratitis, and corneal transplant rejection. Cornea 2000;19:625-43. DOI: https://doi.org/10.1097/00003226-200009000-00008
Abdelfattah NS, Amgad M, Zayed AA, et al. Clinical correlates of common corneal neovascular diseases: a literature review. Int J of Ophthalmol 2015;8:182-93.
Fasciani R, Mosca L, Giannico MI, Ambrogio SA, Balestrazzi E. Subconjunctival and/or intrastromal bevacizumab injections as preconditioning therapy to promote corneal graft survival. Int Ophthalmol 20155;35:221-7. DOI: https://doi.org/10.1007/s10792-014-9938-4
Mimouni M, Ouano D. Initial outcomes of mitomycin intravascular chemoembolization (MICE) for corneal neovascularization. Int Ophthalmol 2022;42:2407-16. DOI: https://doi.org/10.1007/s10792-022-02240-6
Seki Y, Toba K, Fuse I, et al. In vitro effect of cyclosporin A, mitomycin C and prednisolone on cell kinetics in cultured human umbilical vein endothelial cells. Throm Res 2005;115:219-28. DOI: https://doi.org/10.1016/j.thromres.2004.09.001
Nuyts RM, Pels E, Greveya EL. The effects of 5-fluorouracil and mitomycin C on the corneal endothelium. Curr Eye Res 1992;11:565-70. DOI: https://doi.org/10.3109/02713689209001812
Zare M, Jafarinasab M-R, Feizi S, Zamani M. The effect of mitomycin-C on corneal endothelial cells after photorefractive keratectomy. J Ophthalmic Vis Res 2011;6:8-12.
Netto MV, Mohan RR, Sinha S, Sharma A, Gupta PC, Wilson SE. Effect of prophylactic and therapeutic mitomycin C on corneal apoptosis, cellular proliferation, haze, and long-term keratocyte density in rabbits. J Refract Surg 2006;22:562-74. DOI: https://doi.org/10.3928/1081-597X-20060601-08
The Collaborative Corneal Transplantation Studies (CCTS). Effectiveness of histocompatibility matching in high-risk corneal transplantation. The Collaborative Corneal Transplantation Studies Research Group. Arch Ophthalmol 1992;110:1392-403. DOI: https://doi.org/10.1001/archopht.1992.01080220054021
Feizi S. Corneal angiogenesis: etiologies, complications and management. In: Simionescu D, Simionescu A, eds. Physiologic and Pathologic Angiogenesis—Signaling Mechanisms and Targeted Therapy. Rijeka, Croatia: InTech; 2017:57-76. DOI: https://doi.org/10.5772/66713
Sella R, Gal-Or O, Livny E, et al. Efficacy of topical aflibercept versus topical bevacizumab for the prevention of corneal neovascularization in a rat model. Exp Eye Res 2016;146:224-32. DOI: https://doi.org/10.1016/j.exer.2016.03.021
Chu H-S, Chen T-C, Hu F-R, Chen W-L. Recurrence of corneal neovascularization associated with lipid deposition after subconjunctival injection of bevacizumab. Cornea 2013;32:1446-53. DOI: https://doi.org/10.1097/ICO.0b013e31825ec407
Cursiefen C, Hofmann-Rummelt C, Küchle M, Schlötzer-Schrehardt U. Pericyte recruitment in human corneal angiogenesis: an ultrastructural study with clinicopathological correlation. Br J of Ophthalmol 2003;87:101-6. DOI: https://doi.org/10.1136/bjo.87.1.101
Shi H, Zhu Y, Xing C, et al. An injectable thermosensitive hydrogel for dual delivery of diclofenac and Avastin® to effectively suppress inflammatory corneal neovascularization. Int J of Pharmaceutics 2022;625:122081. DOI: https://doi.org/10.1016/j.ijpharm.2022.122081
Pillai CT, Dua HS, Hossain P. Fine needle diathermy occlusion of corneal vessels. Invest Ophthalmol Vis Sci 2000;41:2148-53.